技術・研究材料紹介(企業広告)
環状ペプチドの簡便かつ高感度な定量化ソリューション
株式会社エービー・サイエックス
環状ペプチドは複雑な3次構造から,高いベースライン干渉とCID(衝突誘起解離)によるフラグメンテーション化が難しく高感度定量が難しい化合物となります.
本テクニカルノートでは,ラット血漿中の環状ペプチドEptifibatideの高感度定量ワークフローについて,ZenoTOF7600システムのZeno SIM, Zeno CID, Zeno EAD(Electron Activated Dissociation)モードによる定量を評価いたしました.
A simple and highly sensitive solution for the quantification of cyclic peptidesFeaturing the ZenoTOF 7600 system and SCIEX OS software
|
This technical note describes a highly sensitive quantification workflow for cyclic peptides in rat plasma using accurate mass spectrometry. Low-level quantification was achieved with outstanding accuracy, precision and linearity. A lower limit of quantification (LLOQ) of 0.4 ng/mL was reached for eptifibatide, a model cyclic peptide, exhibiting a more than three-fold improvement in LLOQ compared to previous methods.1-3 Overall, a five-fold signal-to-noise (S/N) improvement was observed using Zeno SIM compared with traditional MS, demonstrating greater sensitivity with the application of the Zeno trap (Figure 1).
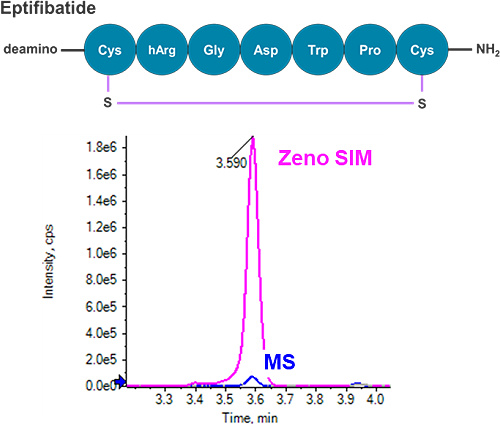
Cyclic peptides are polypeptides in the configuration of a ring formed by chemically stable bonds, such as disulfide bonds between two cysteine residues. Cyclic peptides have been identified as critical therapeutic candidates given their structural stability and conformational rigidity. The stability of a cyclic bond enhances biological activity compared to a linear peptide. As a result, cyclic peptides have emerged as successful therapeutic species in relation to cardiovascular diseases.4
With emerging interest in the advancement of cyclic peptide therapeutics, there is an equivalent drive toward developing highly robust and sensitive quantitative methods.
However, current bioanalytical methods for the quantification of cyclic peptides in biological matrices still present challenges. For LC-MS-based methods, high baseline interference and resistance to collision induced dissociation (CID), given the complex tertiary structure of cyclic peptides, have an impact on overall sensitivity.
In this technical note, eptifibatide was selected as a model analyte to evaluate the quantitative performance of cyclic peptides on the ZenoTOF 7600 system. Zeno SIM, Zeno CID and Zeno electron activated dissociation (EAD) modes were evaluated for quantification. The Zeno SIM approach enabled low-level quantification of cyclic peptides compared to the Zeno CID and Zeno EAD approaches. In addition, the Zeno SIM workflow facilitated an overall reduction in method development time with minimal ion path tuning, offering a simple quantitative workflow for cyclic peptides.
Key features for cyclic peptide quantification using the ZenoTOF 7600 system and SCIEX OS software
- A five-fold S/N improvement using Zeno SIM compared with traditional MS for sensitive quantification of hard-to-fragment cyclic peptides
- A simplified method development paradigm for cyclic peptide quantification when using the Zeno SIM methodology
- Low-level quantification of cyclic peptides in rat plasma with exceptional linearity, precision and accuracy
- A single platform for streamlined data acquisition, processing and management with SCIEX OS software
Methods
Sample preparation: The rat plasma was protein precipitated and the supernatant was diluted 1:1 (v/v) by water and served as the processed biological matrix. Eptifibatide and a labeled cyclic peptide as an internal standard (IS) were spiked into the processed rat plasma. The concentration of the IS in the solution was 10 ng/mL. Serial dilution with processed plasma was performed to create the calibration curves for analysis.
Chromatography: The separation was performed at a flow rate of 0.3 mL/min using an ExionLC system. A HALO BioClass Peptide ES-C18 column (2.1 x 50 mm, 2.7 µm, 160 Å) was used for separation. The column oven temperature was set to 40ºC. The mobile phase A consisted of 0.1% formic acid in water, while the mobile phase B was composed of 0.1% formic acid in acetonitrile. A volume of 20 µL was injected for analysis.
Chromatographic conditions are summarized in Table 1.
| Time (min) | Mobile phase A (%) | Mobile phase B (%) |
|---|---|---|
| 0.0 | 95.0 | 5.0 |
| 1.0 | 95.0 | 5.0 |
| 3.0 | 75.0 | 25.0 |
| 4.0 | 40.0 | 60.0 |
| 4.2 | 10.0 | 90.0 |
| 5.2 | 10.0 | 90.0 |
| 5.5 | 95.0 | 5.0 |
| 6.5 | 95.0 | 5.0 |
Mass spectrometry: Data were acquired in positive mode using Zeno CID, Zeno SIM and Zeno EAD on a ZenoTOF 7600 system. Zeno SIM is a Zeno MRMHR workflow with minimal CE to enable monitoring of the intact precursor ion. An MRMHR workflow was applied for all MS modes examined. The source was operated in positive ion mode. Collision energy (CE) and other source and MS parameters were optimized for eptifibatide.
A summary of the source and MS parameters and the Zeno trap settings is displayed in Table 2.
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Curtain gas | 35 psi | Source temperature | 650°C |
| Ion source gas 1 | 30 psi | Ion source gas 2 | 80 psi |
| CAD gas | 11 | Ion spray voltage | 4000 V |
| Q1 resolution | Low | ZOD threshold | 20,000 cps |
For Zeno CID and Zeno EAD, a suitable m/z range of fragment ions was monitored. EAD parameters such as ETC, electron KE and reaction time were optimized for eptifibatide, as shown in Table 3.
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Electron beam current | 4750 nA | ETC | 100 |
| Electron KE | 15 | Reaction time | 5 ms |
Data processing: Zeno CID, Zeno SIM and Zeno EAD data were processed using the Analytics function in SCIEX OS software 2.0 with the MQ4 integration algorithm. A 1/x2 weighting was used for quantification.
Overview of the cyclic peptide workflow
Eptifibatide was used as a model analyte to evaluate the quantification of cyclic peptides on the ZenoTOF 7600 system.
Eptifibatide was spiked into processed rat plasma at concentrations ranging from 0.4 ng/mL to 423 ng/mL. The precursor ion for eptifibatide, m/z 832.3215, was applied for quantitative workflows involving Zeno CID, Zeno EAD and Zeno SIM.
For Zeno CID, fragment ions were monitored in the m/z 400 to m/z 900 range. The Zeno EAD workflow captured fragment ions in the m/z 100 to m/z 900 range. For quantification using Zeno CID and Zeno EAD, the most intense fragment ions were summed for optimal assay sensitivity.
Evaluation of quantitative MS modes for cyclic peptides
In this workflow, quantification of cyclic peptides was compared using Zeno SIM, Zeno CID and Zeno EAD. All calibration points were measured in triplicates. The LLOQ was determined based on the requirements that the %CV of the average of the concentration must be below 20% and the accuracy must be between 80% and 120%. For the concentrations above the LLOQ, the %CV of the mean of the calculated concentration was required to be below 15% and the accuracy was required to be between 85% and 115%.
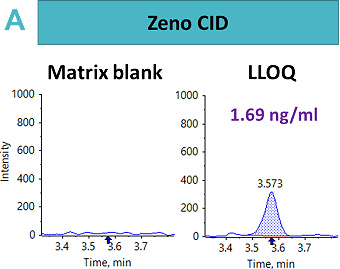
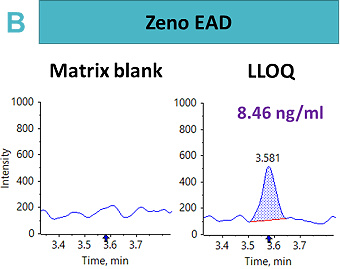
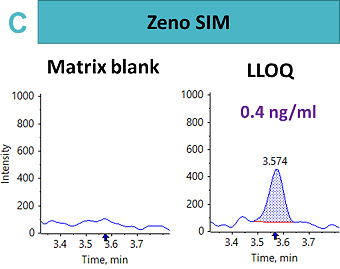
An LLOQ of 1.69 ng/mL, 8.46 ng/mL and 0.4 ng/mL was achieved using the Zeno CID, Zeno EAD and Zeno SIM modes, respectively (Figure 2). In this case, the best sensitivity was reached using Zeno SIM with up to a four-fold improvement in LLOQ compared with Zeno CID and a 21-fold improvement in LLOQ compared with Zeno EAD.
This suggests that for complex peptides, such as those with disulfide-bridged modifications, resistance to fragmentation may cause a loss in sensitivity for quantitative assays, unless analyzed with a high duty cycle triple quadrupole system such as the SCIEX 7500 system.5 With Zeno SIM mode, the precursor ion was used for quantification with the application of the Zeno trap, which enhances overall sensitivity. In addition to the benefits of the Zeno trap in Zeno SIM mode, the workflow offers reduced method development time with less ion path tuning. As a result, the Zeno SIM approach presents the most sensitive and simple workflow for the quantification of cyclic peptides.
Quantitative performance of Zeno SIM
For Zeno SIM, the overall linear range covered 0.42 ng/mL to 423 ng/mL. A linear dynamic range (LDR) was confirmed to be three orders of magnitude (Figure 3).
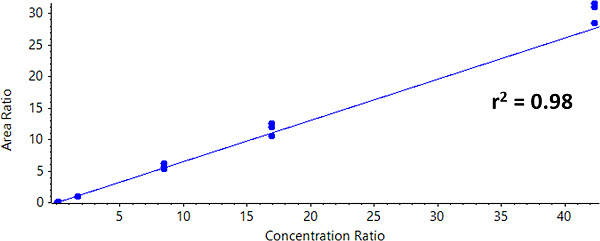
Calculated concentrations for each calibration point were within ±15% of the nominal value, even at the level of the LLOQ (Table 4). As shown in Table 4, the precision was less than 13%, demonstrating high reproducibility.
The quantitative results-including accuracy, precision and linearity-for the data acquired using Zeno CID and Zeno EAD were comparable to Zeno SIM (data not shown).
| Concentration (ng/mL) | Accuracy (%) | Percent CV (%) |
|---|---|---|
| 423.29 | 109.9 | 5.52 |
| 169.31 | 105.76 | 8.61 |
| 84.66 | 104.08 | 7.77 |
| 16.93 | 90.81 | 2.95 |
| 1.69 | 85.69 | 2.1 |
| 0.42 | 103.76 | 12.75 |
Conclusions
- A five-fold S/N improvement was achieved using Zeno SIM compared with traditional MS for sensitive quantification of hard-to-fragment cyclic peptides.
- Development of a simple workflow for cyclic peptide quantification was accomplished with less method development time using the Zeno SIM approach.
- Low-level quantification of cyclic peptides in rat plasma was reached with exceptional linearity, precision and accuracy.
- An LLOQ of 0.4 ng/mL was reached for eptifibatide, demonstrating a more than three-fold improvement in LLOQ compared to previous methods.1-3
- A single platform for streamlined data acquisition, processing and management with SCIEX OS software was presented.
References
- Chen, J. et al. (2018). J. Pharm. and Biomed. Analysis, 159, 217-223.
- Liu, J. et al. (2009). J. Chrom. B, 877(5-6), 527-532.
- Zhou, Z. et al. (2010). J. Chrom. B, 878(23), 2094-2100.
- Das BB, Solinger R (2009). Cardiovasc. Hematol. Agents Med. Chem. 7(1), 29-42.
- Improved LC-MRM quantification sensitivity for cyclic peptides from the natriuretic peptide family. SCIEX technical note, RUO-MKT-02-11883-A.
技術的なご相談・ご質問はこちら
TEL:0120-318-551 (受付時間 平日9:00~17:30)
E-mail:jp_sales@sciex.com
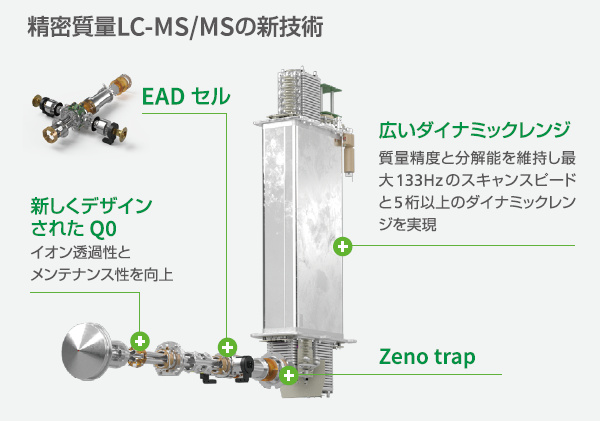
新しい高分解能質量分析装置
あらゆる化合物分析に 新たな構造情報を
SCIEX ZenoTOF 7600システム

2つの特徴
多様な化合物の構造推定が可能に【EAD:電子励起解離】
電子エネルギーが調整可能なEADセルで,低分子化合物からタンパク質まで,解析に必要なフラグメントイオンを取得可能.
感度が従来より5-20倍向上【Zeno trap pulsing】
Zeno trapの使用で,MS/MSデューティーサイクルを向上し,90%以上のTOFへのイオン注入が可能に.
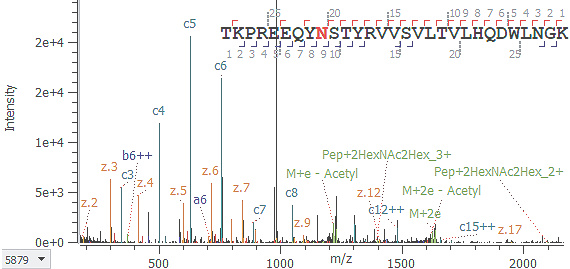
EAD:
ペプチド配列と糖結合位置情報を取得(c9++イオン)
CID:
糖が遊離し,ペプチド骨格の情報のみ
 株式会社エービー・サイエックス
株式会社エービー・サイエックス
本社:〒140-0001東京都品川区北品川 4-7-35 御殿山トラストタワー 21F
TEL:0120(318)551 FAX:0120(318)040
大阪:〒531-0072大阪府大阪市北区豊崎 3-19-3 ピアスタワー 3F
www.sciex.jp Email: jp_sales@sciex.com
The SCIEX clinical diagnostic portfolio is For In Vitro Diagnostic Use. Rx Only. Product(s) not available in all countries. For information on availability, please contact your local sales representative or refer to https://sciex.com/diagnostics. All other products are For Research Use Only. Not for use in Diagnostic Procedures. Trademarks and/or registered trademarks mentioned herein, including associated logos, are the property of AB Sciex Pte. Ltd. or their respective owners in the United States and/or certain other countries. Echo and Echo MS are trademarks or registered trademarks of Labcyte, Inc. in the United States and other countries, and are being used under license. The images shown may be for illustration purposes only and may not be an exact representation of the product and/or the technology.
Plates are available from Beckman Coulter Life Sciences.© 2023 DH Tech. Dev. Pte. Ltd.


